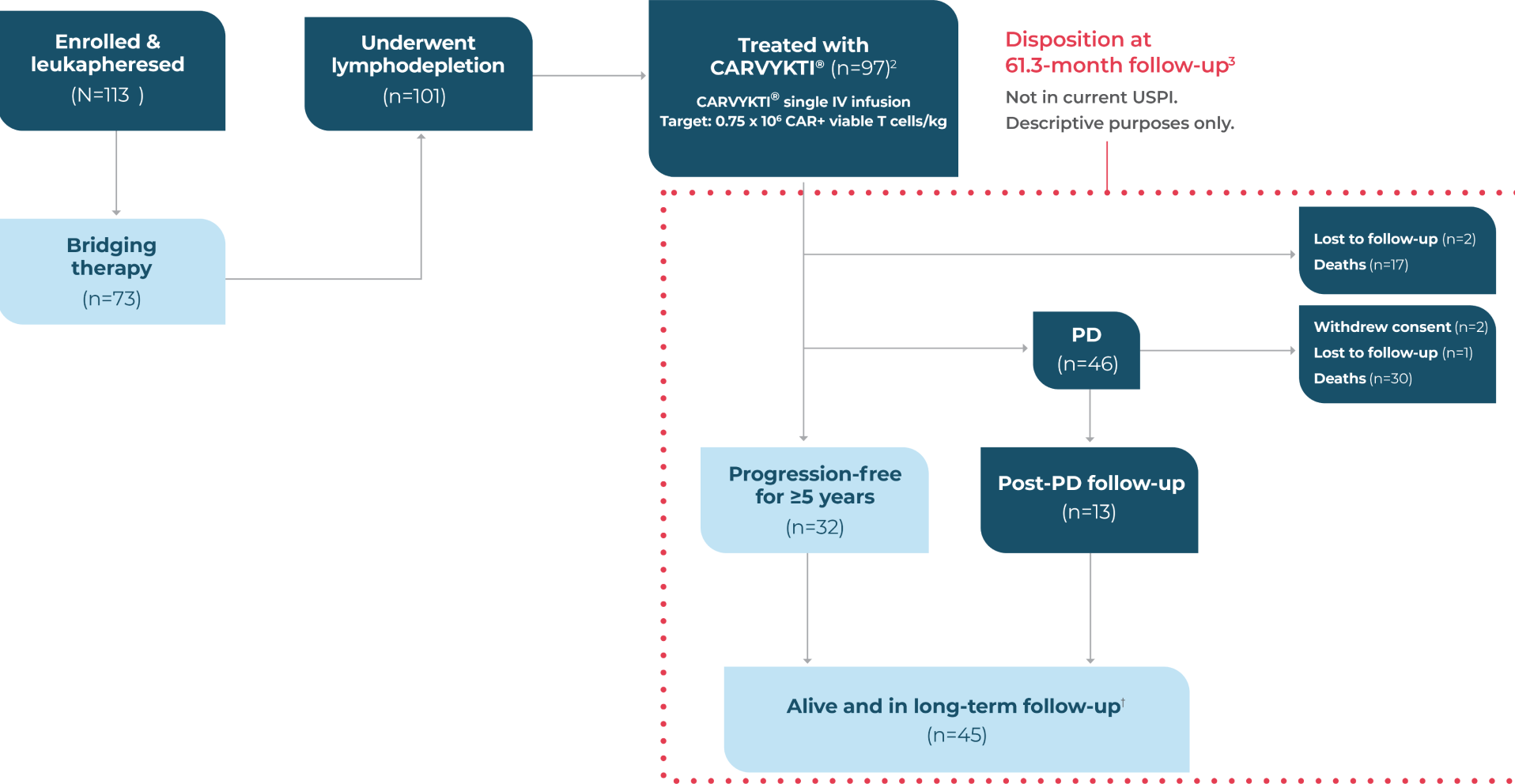
CARTITUDE-1 STUDY DESIGN1,2
Phase 1b/2, open-label, multicenter trial of 97 adult patients with relapsed or refractory multiple myeloma
Primary objectives1,2
PHASE 1b
Characterize safety and confirm Phase 2 dose
PHASE 2
Evaluate efficacy:
- ORR (primary endpoint)
- sCR, CR, VGPR, DOR, PFS, OS (select secondary endpoints)

PHASE 1b
Characterize safety and confirm Phase 2 dose
PHASE 2
Evaluate efficacy:
- ORR (primary endpoint)
- sCR, CR, VGPR, DOR, PFS, OS (select secondary endpoints)
CAR+=chimeric antigen receptor-positive; CR=complete response; DOR=duration of response; IV=intravenous; ORR=overall response rate; OS=overall survival; PD=progressive disease; PFS=progression-free survival; sCR=stringent complete response; VGPR=very good partial response.
*At median 61.3-month follow-up.
CARTITUDE-1 INCLUDED HEAVILY PRETREATED RRMM PATIENTS1,2
Key Eligibility Criteria
- Diagnosis of MM per IMWG criteria, with measurable disease
- ≥3 previous LoT (or double refractory to a PI and an immunomodulatory agent)
- Previous treatment with a PI, an immunomodulatory agent, and an anti-CD38 monoclonal antibody
- Disease progression per IMWG criteria within 12 months of last LoT
- ECOG PS 0-1*
Exclusion Criteria
- Known active or prior history of significant CNS disease, including CNS multiple myeloma
- Plasma cell leukemia
- Allogeneic stem cell transplant within 6 months before apheresis or ongoing treatment with immunosuppressants
- Creatinine clearance <40 mL/min
- Absolute lymphocyte concentration <300/μL
- Absolute neutrophil count <750 cells/mm3
- Platelet count <50,000/mm3
- Hepatic transaminases >3x the upper limit of normal
- Cardiac ejection fraction <45%
- Active serious infection
- Prior treatment with CAR-T directed at any target
- Prior therapy that is targeted to BCMA
BCMA=B-cell maturation antigen; CAR-T=chimeric antigen receptor-T cell; CD38=cluster of differentiation 38; CNS=central nervous system; ECOG PS=Eastern Cooperative Oncology Group performance status; IMWG=International Myeloma Working Group; LoT=line(s) of therapy; MM=multiple myeloma; PI=proteasome inhibitor.
*ECOG performance status at baseline was 2 in 4% of patients.2
cartitude-1 study:
select baseline characteristics (n=97)1,2
| DEMOGRAPHICS | |
|---|---|
| Age, range (median) | 43-78 years (61) |
| Male (n) | 59% (57) |
| African American (n) | 18% (17) |
| ECOG performance status 0 (n) | 40% (39) |
| ECOG performance status 1 (n) | 56% (54) |
| ECOG performance status 2 (n) | 4% (4) |
| DISEASE | |
|---|---|
| High-risk cytogenetic profile (n)* | 24% (23) |
| Extramedullary plasmacytomas ≥1 (n) | 13% (13)† |
| Tumor BCMA expression ≥50% (n) | 92% (57)‡ |
| CrCl <45 mL/min (n) | 3% (3) |
| PRIOR TREATMENTS | |
|---|---|
| Time since diagnosis, median | 5.9 years |
| Prior LoT, median (range) | 6 (3-18) |
| Triple-class exposed (n)§ | 100% (97) |
| Penta-exposed (n)|| | 84% (81) |
| Triple-class refractory (n)§ | 88% (85) |
| Penta-refractory (n)|| | 42% (41) |
| Refractory to LoT (n)¶ | 99% (96) |
| Prior autologous stem cell transplant (n) | 90% (87) |
| Previous allogeneic stem cell transplant (n) | 8% (8) |
BCMA=B-cell maturation antigen; CD38=cluster of differentiation 38; CrCl=creatinine clearance; del=deletion; ECOG=Eastern Cooperative Oncology Group; LoT=line(s) of therapy; t(14;16)=translocation 14;16; t(4;14)=translocation 4;14.
BCMA=B-cell maturation antigen; CD38=cluster of differentiation 38; CrCl=creatinine clearance; del=deletion; ECOG=Eastern Cooperative Oncology Group;
LoT=line(s) of therapy; t(14;16)=translocation 14;16; t(4;14)=translocation 4;14.
*Based on the presence of del(17p), t(14;16), or t(4;14).1,2
†Additional 6 patients had a soft-tissue component of a bone-based plasmacytoma (total plasmacytomas, 19.6%).4
‡Denominator n=62, the number of evaluable samples; BCMA expression detected in all evaluable samples.4
§≥1 proteasome inhibitor, ≥1 immunomodulatory agent, and 1 anti-CD38 monoclonal antibody.2
‖≥2 proteasome inhibitors, ≥2 immunomodulatory agents, and 1 anti-CD38 monoclonal antibody.2
¶Two patients were refractory to other anti-CD38 antibodies.2
Median follow-up: 28 months
95% OF PATIENTS ACHIEVED DEEP RESPONSES WITH CARVYKTI®
In CARTITUDE-11,2
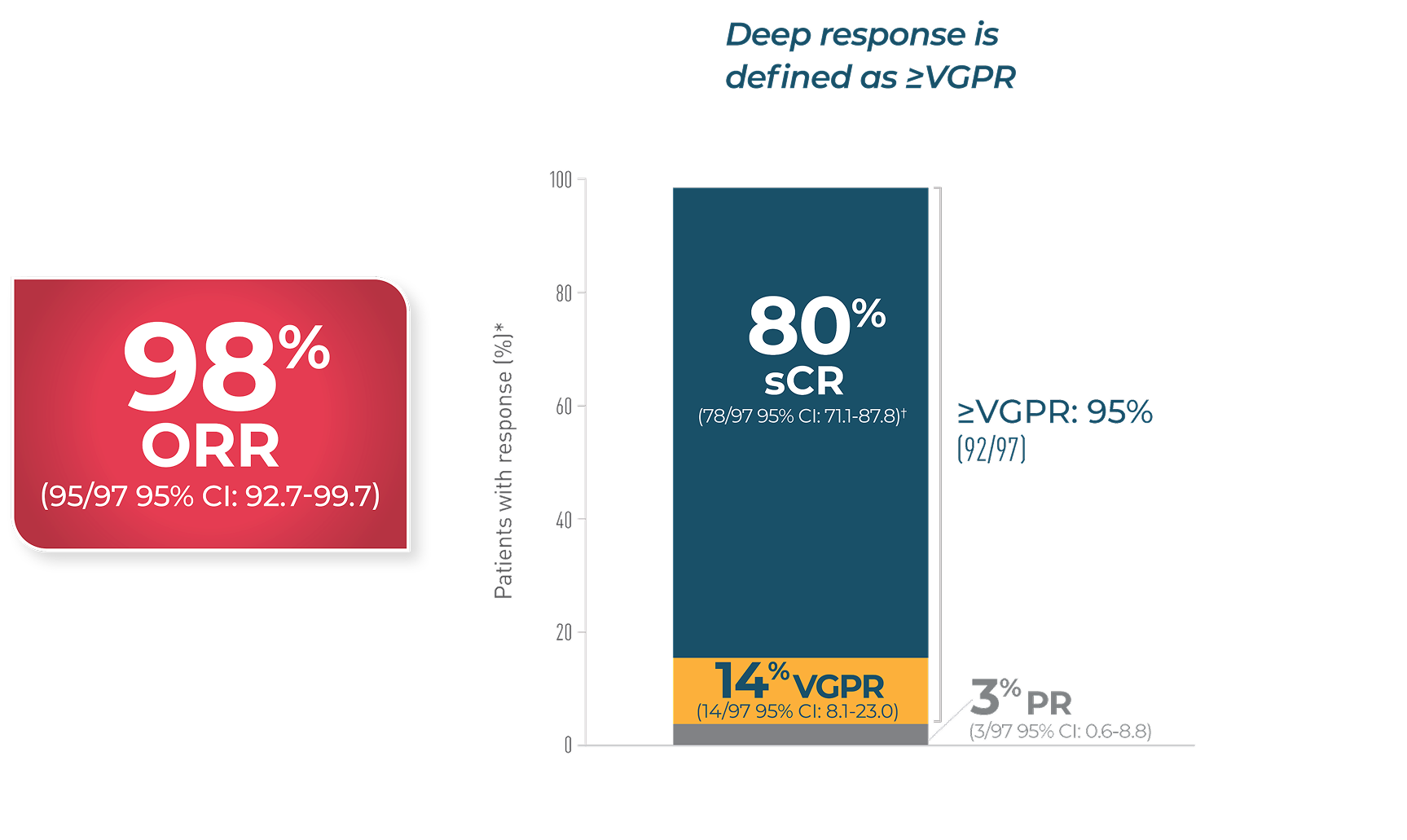

Percentages rounded to nearest whole number and may not add up due to rounding.
Cl=confidence interval; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PR=partial response;sCR=stringent complete response; TTR=time to response; VGPR=very good partial response.
*Based on a median duration of follow-up of 28 months.1
†All complete responses were sCRs.1
Median follow-up: 28 months
DURABLE RESPONSES
In CARTITUDE-11*


CI=confidence interval; mDOR=median duration of response; NE=not estimable.
*Based on a median duration of follow-up of 28 months.1
Median follow-up: 28 months
Time to Response
In CARTITUDE-11


*Based on a median duration of follow-up of 28 months.1
Learn More About CARVYKTI®